Nitrogen
Influence of N on Plant Growth and Development
N is taken up as NH4+ or NO3-.
It is essential as a component of many different biomoledules such as proteins,
nucleic acids and chlorophyll. Deficiency results in
chlorosis and poor growth. However, oversupply causes
rank but abnormal growth and poor quality.
Origin and Distribution of Nitrogen
Most N is atmospheric. There is 10 to 20 x as much N in soil as in vegetative cover. Most soil N is in organic combinations and N comprises about 5 % of soil organic matter. Only about 1 or 2 % of the soil N is inorganic.
Immobilization and Mineralization
Mineralization is the conversion of organic-N to inorganic-N. About 2.5 % of the organic-N in soil mineralized annually. This represents a major source of N for plant growth. Immobilization is the incorporation of inorganic-N into organic compounds. If the C / N is high, immobilization > mineralization.
NH4+ Fixation
Entrapment of NH4+ between adjacent tetrahedral sheets of neighboring layers of 2:1 minerals. The tendency for fixation follows the sequence, vermiculite > illite > smectites. Fixed NH4+ is slowly released.
NH3 Volatilization
NH4+ + OH- <=> H2O + NH3
Conditions favoring
Conditions opposing
volatilization
volatilization
______________________________________
High pH
Low pH
Low CEC
High CEC
Surface application
Incorporation in soil
Dry soil
Moist soil
High temperature
Cool temperature
Nitrification
Microbial oxidation of NH4+
2NH4+ + 3O2 ->
2NO2- + 4H+ + 2H2O + energy
2NO2- + O2
-> 2NO3+ + energy
Nitrification is carried out by autotrophic bacteria
Step 1 Nitrosomonas
Step 2 Nitrobacter
As seen from the above reaction, nitrification acidifies soil.
Soil environmental conditions affecting nitrification include
High NH3 concentration, which inhibits
the process
O2 required since nitrification is aerobic
Moist conditions favor nitrification but not so
wet as to affect O2 availability
Optimum temperature range is about 25 - 35 C
Good soil fertility also favors nitrification
There are chemical inhibitors that reduce the activity
of Nitrosomonas and, therefore, slow nitrification and the loss
of N as NO3- by leaching or denitrification.
Nitrification is typically
rapid.
NO3- Leaching
This is undesirable with respect to plant growth since it is a loss of N from the soil. Nitrate movement to ground and surface water also poses health and environmental risks. Methemoglobinemia (blue baby syndrome) is due to reduction of NO3- to NO2-, which reduces the capacity of hemoglobin to carry O2. Enrichment of surface waters with NO3- may lead to eutrophication, especially of marine systems.
Factors influencing NO3- leaching include
Soil water drainage
NO3- concentration
Denitrification
Reduction of NO3- to NO, N2O or N2
NO3- -> NO2-
-> NO -> N2O -> N2
volatile losses
Denitrifying organisms include facultative anaerobes such as Pseudomonas and Bacillus that are heterotrophic and autotrophic Thiobacillus denitrificans.
Factors affecting denitrification
Presence of NO3-
Oxidizable substrates (for heterotrophs)
Anaerobic conditions
Optimum temperature 25 - 35 C
Low pH < 5 inhibits denitrification
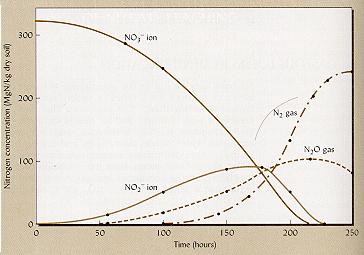
Example of denitrification
kinetics.
Denitrification occurs in wet soils like riparian zones; wetlands and rice fields; even in set areas of upland agricultural soils (spatially and temporally variable, up to 60 kg / ha annually).
Nitrification and denitrification
in a
paddy soil.
Denitrification contributes to acid deposition from HNO3 formed from NO and N2O. Also, N2O is a greenhouse gas.
Biological N Fixation
N2 + 6H+ + 6e -> 2NH3
The NH3 is incorporated into amino acids.
Biological N-fixation is carried out by certain bacteria, actinomycetes,
and
cyanobacteria. It has been estimated that
139,000,000 Mg N is annually fixed in terrestrial
systems.
Nitrogenase is the enzyme complex involved. It consists of two proteins. The smaller one supplies e-s and larger traps N2 and the larger supplies electrons for reduction. Since the reaction requires energy and it is aided by association with plants which supply photosynthetic products. Interestingly, nitrogenase is degraded by O2 and isprotected from O2 by leghemaglobin. The formation of root nodules that contain the N-fixing bacteria is inhibited by soil NO3-. On the other hand, good Mo, Fe, P and S fertility is needed for N-fixation.
Growth with and without
N-fixing organisms.
Symbiotic Fixation with Legumes
Rhizobium and Bradyrhizobium are the genera of bacteria involved. These form nodules on roots of legumes. The symbiosis is specific between legume and bacteria species. To ensure root nodulation one can inoculate if the right species is not present.
Nodulated root.
Symbiotic Fixation with Nonlegumes
Some form nodulated associations. Actinomycetes of the genera Frankia are involved.
There are also non-nodulated associations, like the association of Anabaena within leaves of Azolla. Another non-nodulated symbiosis involves N-fixing organisms living in close, but external, association with plant roots in the rhizosphere.
Sulfur
This elemental is a component of certain amino acids and vitamins. Deficiencies in S result in chlorosis and stunted growth.
Sources of S include organic S; soil minerals such as CaSO4 (arid regions), FeS (formed under reducing conditions) and, most commonly, SO4- adsorbed to colloids; and atmospheric forms.
S Oxidation and Reduction Reactions
Mineralization of organically bound S releases incompletely oxidized forms of S.
Oxidation to SO4- is largely a biological process
H2S + 2O2 -> H2SO4
2S + 3O2 + 2H2O -> 2H2SO4
Oxidation is carried out by autotrophic Thiobacillus.
Reduction occurs under anaerobic conditions.
SO4-2 + 8H+ + 8e -> S2- + 4H2O
It is carried out by Desulfovibrio and is coupled with the oxidation of organic matter. The sulfide produced is subject to precipitation.
S2- + Fe2+ -> FeS
Environmental Acidification Problems due to Inorganic Sulfur
Acid sulfate soils
Mined soils
Oxidation of FeS and FeS2 leads to very low soil pH. Once soil containing reduced S is drained and aerated (or minerals containing reduced S are excavated), reduced S is subject to oxidation.
4FeS + 9O2 + 4H2O -> 2Fe2O3 + 4SO42- + 8H+
Acid deposition on forest soils
H2SO4 + HNO3
These loadings of H+, in addition to acidity due to carbonic acid and organic and mineral acids from organic matter decomposition, accelerate the natural leaching loss of nutrients.