Yield reduction of several crops as a function of the electrical conductivity
(EC) of soil.
Mostly a problem in arid and semiarid regions where soils have high base saturation but soluble salts may also be high where irrigation water adds salt and drainage is poor. A high concentration of Na poses special problems because of the tendency of Na to disperse soil colloids.
Development of Saline and Sodic Soils
Natural salinity
Largely due to Ca2+, Mg2+, K+, Na+, Cl- and SO4- occurs where there is insufficient rainfall to leach naturally occurring salts from the upper profile. Especially high concentrations may occur at low positions in certain landscapes where laterally moving ground water contains heavy load of salt. Called saline seeps.
Irrigation induced salinity
Use of surface or ground water containing
dissolved salts for irrigation adds salt. Evaporation from soil surface
leads to accumulation of salt at or near the soil surface resulting in
a saline soil. If the Na, though not total salt, concentration is
high a sodic soil is formed. These problems are avoided if there
is adequate drainage.
Effects of Salinity on Plant Growth
Osmotic effect
Salinity decreases soil water potential so that the plant is drought stressed at lower matric potential (or soil water content). At even lower water content (increased salt concentration), exosmosis may occur. Salinity effect is typically worse on young plant than mature plant and may inhibit seed germination. Salt tolerance to varies with species.
Yield reduction of several
crops as a function of the electrical conductivity
(EC) of soil.
Measuring Salinity and Sodium Content
Soluble salt concentration
Exchangeable sodium percentage
Sodium adsorption ratio
Soluble salt concentration
Electrical conductivity EC of water (soil water) increases with increasing concentration of electrolytes. The EC is directly related to the ionic strength of the soil solution and the ionic strength depends on the concentration (and charge) of electrolytes. So EC is a measure of salt concentration. The units of EC are decisiemens per m (dS /m). It is measured in laboratory using a soil water extract. It can also be measured in the field, either directly or indirectly, however, less accurately.
Exchangeable Na percentage ESP
ESP = (exchangeable Na+ cmolc / kg) x 100 % / (total exchangeable cations cmolc / kg)
Soils with high ESPs have high pHs
ESP = 15 % pH = 8.5
Higher ESPs pH > 10
Sodium adsorption ratio SAR
SAR = [Na+ ] / ([Ca2+] + [Mg2+])½
The SAR is highly correlated to ESP and
since it is more easily measured than ESP, it is more often used to characterize
the Na content of soils.
Classification of Saline and Sodic Soils
Type EC SAR
Saline > 4.0 < 13
Sodic < 4.0 > 13
Saline-sodic > 4.0
> 13
Saline soils
Salt concentration as measured by EC is sufficiently high to adversely affect growth of most plants. But adsorbed Na+ sufficiently low and Ca2+ and Mg2+ high that soil is not dispersed. White alkali soil
Sodic soils
Level of soluble salts is low EC < 4.0
dS / m but SAR > 13 and ESP > 15 %. Also,
pH 8.5. The high exchangeable Na+
disperses soil colloids and drastically reduces hydraulic conductivity.
Plant growth may be affected by high level of Na+, HCO3-
and CO32- and high pH.
Dispersed organic colloids appear as a
dark surface deposit. Black alkali soil
Saline-sodic soils
Characteristics of both saline and sodic
soils. But high concentration of soluble salts limits the tendency of soil
high in exchangeable Na+ to disperse. However, saline-sodic
soils may become sodic if the soluble salts leached.
Reclaimation of Saline Soils
Flush soluble salts from soil profile. This requires irrigation water that is low in soluble salts together with good soil drainage.
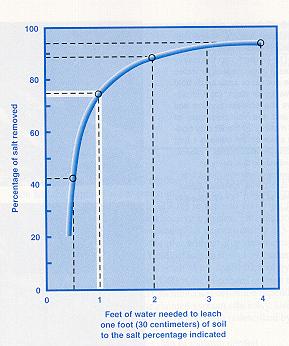
Crudely, about 30 cm of
water
is needed to remove about
3/4
of soluble salts from each
30 cm depth of salt-affected
soil.
To avoid accumulation of salt in the root
zone, irrigation water in excess of that needed to supply water for plant
growth is added. This excess amount is called the leaching requirement.
Reclaimation of Saline-Sodic and Sodic Soils
Cannot leach excess salts from saline-sodic soil because reduced ionic strength of soil solution in presence of high Na+ will lead to soil dispersion. The high concentration of Na+ must first be reduced. Sodium is replaced by Ca2+ or H+.
Add gypsum to reduce adsorbed Na+ and carbonates
2NaHCO3 + CaSO4 -> CaCO3 + Na2SO4 + H2O + CO2
2XNa + CaSO4 -> XCa + Na2SO4
Similarly, by addition of S or H2SO4
2NaHCO3 + H2SO4 -> Na2SO4 + 2H2O + 2CO2
2XNa + H2SO4 -> 2XH + Na2SO4
Drainage is improved and hydraulic conductivity is increased. Soluble salts are then leached from the profile.