Soil Colloids
General Properties
Soil colloids are size and. therefore, have a large
surface area per unit mass. Soil colloids also carry electrostatic charges
(- and +) that are balanced by adsorbed cations and anions.
Four general types
Layer silicates which consist of thin layers
of repeated structural units. These are the dominant clay minerals in temperate
regions.
Amorphous silicates that form from volcanic
ash.
Al and Fe oxides which may be crystalline
or amorphous. These are common in subtropical and tropical regions.
Organic (humus) which are 3D noncrystalline
polymers present in all soils.
Layer Silicates
General structure
Composed of alternating sheets of Si tetrahedra and
Al (or Mg) octahedra. The octahedral sheet is called dioctahedral
if Al is the central metal atom or
trioctahedral if it is Mg. The
Si tetrahedal sheet is chemically bonded to the one or two adjacent Al
(or Mg) octahedral sheet(s) via shared oxygen atoms.
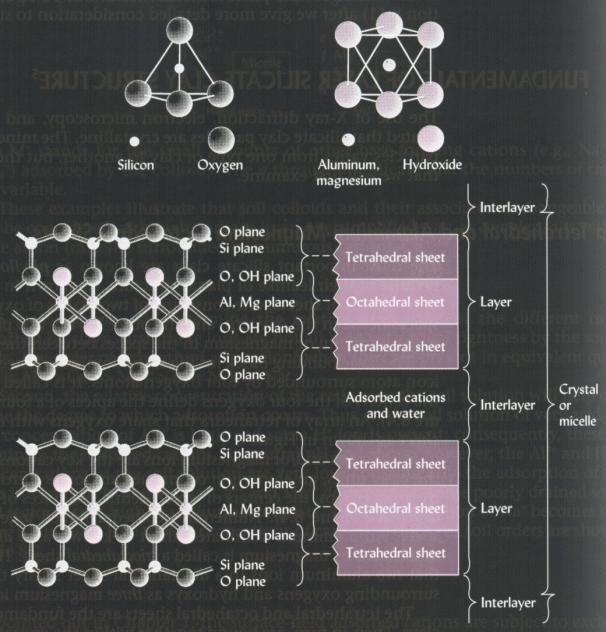
Basic units are the Si tetrahedron
and the Al (or Mg) octahedron.
Many
tetrahedra are linked together
to form
a Si tetrahedral sheet and
many
octahedra are similarly
linked to from
an octahedral sheet.
In turn, these
different sheets are bonded
together to
form crystalline units.
Source of electrostatic charge
Isomorphic substitution
Al3+ for Si4+ in the tetrahedral
sheet and Mg2+ for Al3+ in the dioctahedral
sheet.
These substitutions occur during formation of the
clay mineral and are permanent to the structure. Both lead to net
negative charge within the crystal lattice that is balanced by adsorbed
cations.
pH-dependent charge
Loss of ionizable H+ from certain sites
on mineral colloids or from certain functional groups in humus leads to
negatively charged sites. Protonation of other sites leads to positively
charged sites. pH-dependent negative charge increases with increasing pH
but pH-dependent positive charge increases with decreasing pH.
Types of layer silicates
1:1
2:1
2:1:1
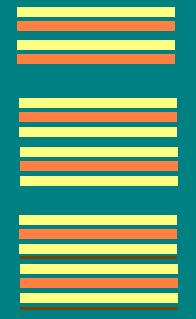
Orientation of tetrahedral
and octahedral sheets in
1:1, 2:1 and 2:1:1layer
silicates.
1:1 layer silicates
These have one Si tetrahedral and one Al octahedral
sheet per crystalline unit.
Adjacent layers (units) are H-bonded together via
sharing of H from octahedral -OH with O of the tetrahedral sheet of adjacent
layer.
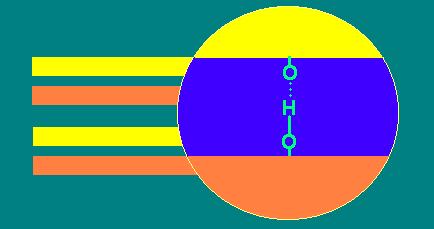
1:1 layer silicates do not expand.
Since adjacent layers are H-bonded together, these
minerals are nonexpanding and exhibit only an external surface area. These
exhibit little plasticity, cohesion or swelling. Also, there is little
isomorphic substitution and the capacity to adsorb cations (cation exchange
capacity, CEC) is low.
Kaolinite, halloysite and dickite are 1:1 layer silicates.
2:1 layer silicates
These have the Al (or Mg) octahedral sheet bonded
to Si tetrahedral sheets on top and bottom. Unlike the 1:1 type minerals,
certain 2:1 types of minerals may expand by adsorption of water between
adjacent 2:1 units. There are three types of 2:1 minerals
Smectite
Vermiculite
Illite
Smectite
The octahedral sheet in smectites is dioctahedral.
Adjacent 2:1 units are weakly held together by cations mutually adsorbed
by each layer. Accordingly, smectites expand upon adsorption of water between
layers and, therefore, exhibit a large total (external + internal) surface
area. These minerals are highly plastic, cohesive and swelling. The CEC
is large due to a high extent of isomorphic substitution, especially in
the dioctahedral sheet.
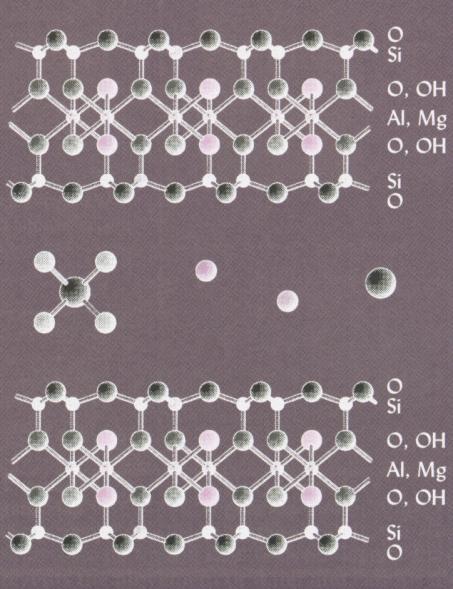
Sections of two units of
a
smectite. Water and
cations
may enter and leave the
interlayer region.

Smectites shrink when dry
and swell when wet.
Smectites include montmorillonite, beidellite and
nontronite.
Vermiculite
The octahedral sheet may be dioctahedral or trioctahedral.
Extensive isomorphic substitution of Al3+ for Si4+
in the tetrahedral layers leads to an even larger CEC than in the smectites.
There is strong affinity for cations (especially adsorbed Mg2+)
briding tetrahedral sheets of adjacent 2:1 layers, leading to limited-expansion.
Illite (or fine-grained mica)
These are chemically altered micas. There is extensive
isomorphic substitution in the Si tetrahedral sheet. Due to geometry of
the substituted tetrahedral sheet, adsorption of K+ at interlayer
positions holds adjacent 2:1 units tightly together.
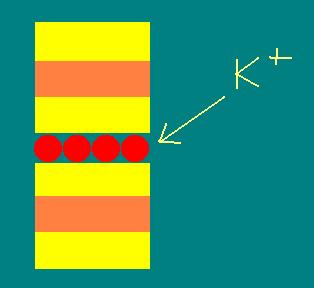
Interlayer K holds adjacent
units of illite
tightly together.
It does not expand.
Therefore, illite is nonexpanding and exhibits a
much smaller total surface area per unit mass than smectites or vermiculites.
The CEC is much less than other 2:1 minerals.
2:1:1 layer silicates
These are also 2:1:1 type minerals that consist
of a Mg octahedral sheet between adjacent 2:1 units. There is little
Al in octahedral sheet, Fe and Mg instead. These minerals are nonexpanding
and exhibit a fairly low CEC similar to illite.
Formation of Soil Colloids
Layer Silicate Clays
These may develop from chemical alterations of primary
minerals such as micas or feldspars. Alternatively, these may precipitate
from soil solution containing dissolved Al and SiO2. The particular
layer silicate that precipitates depends on relative stability in the prevailing
soil chemical environment. In general, the 2:1 minerals are less stable
under hot and wet conditions than are the 1:1 minerals.
Al and Fe oxides
In a general sequence, these minerals are the end
products of a sequence leading from primary minerals to 2:1 clays to 1:1
clays. Therefore, the Al and Fe oxides are stable mineral colloids.
Examples include Al(OH)3 (gibbsite), FeOOH
(goethite) and Fe2O3 (hematite).
Amorphous colloids
Weathering of volcanic ash releases substantial quantities
of dissolved Al and SiO2 which precipitate as amorphous allophane.
Organic colloids
Complex organic molecules formed by microbial tranformation
of biomolecules
Distribution of Clay Minerals
Varies within the profile as well as geographically
depending upon climate (internal and external) and parent material. The
different soil orders, therefore, tend to differ in clay mineralogy.
Order
Dominant Clay Minerals
Aridisols
2:1
Vertisols
2:1 smectites (shrink-swell)
Mollisols
2:1 > 1:1
Alfisols
2:1 = 1:1
Ultisols
1:1 > Al and Fe oxides > 2:1
Spodosols
Al and Fe oxides = 1:1
Oxisols
Al and Fe oxides > 1:1
Note that the mineralogy of the sequence of increasingly
weathered soil orders, Alfisols, Ultisols and Oxisols, is reflected in
the dominant clay mineralogy of these orders.
More on Electrostatic Charges
Permanent
Negative charges arise from isomorphic substitution
in tetrahedral or octahedral layers. Limited positive charges may also
arise from substitution of Al3+ for Mg2+ in trioctahedral
sheet of chlorite and some vermiculites.
The difference between permanent negative and positive
charges gives net permanent charge
pH-dependent
Negative charge
Other than permanent charge, the magnitude and sign
of electrostatic charge on soil colloidal particles is pH-dependent. Negative
pH-dependent charge arises from the ionization of H from -OH groups on
surfaces or at edges of silicate clays and Al and Fe oxides. Ionization
of H from -OH, -COOH and aromatic -OH of humic colloids also generates
localized negative sites. Neutralization of positive charge associated
with adsorbed Al3+ (or hydrolyzed species) also effectively
increases CEC.
Positive charge
Protonation of -OH to give -OH2+
leads to positive charges. Common for Al and Fe oxides and 1:1 silicate
clays.
Electrostatic charge on the mixture of inorganic
and organic colloids in soil includes both negative and positive charges.
pH-dependent negative charge
increases
with increasing pH but pH-dependent
positive charge decreases
with increasing pH.
Cation Exchange
There is thermodynamic equilibrium between the concentration
of cations in solution and adsorbed on soil colloids. The below example
show stoichimetric exchange of solution phase K+ for adsorbed
Ca2+ (represented as, XCa2+).
XCa2+ + 2K+ <=>Ca2+
+ 2XK+
The distribution of Ca2+ and K+
between solution and adsorbed phases depends on the exchange selectivity
coefficient.
K= [Ca2+][XK+]2
/ [XCa2+][K+]2
Note that if the concentration of K+ in
solution were increased, the concentration of adsorbed K+ would
increase such that equilibrium was maintained and visa versa. Also, the
colloid typically exhibits greater preference for one of the pair of adsorbed
cations. In general, the order of affinity for cation adsorption follows
Al3+ = H+ > Ca2+
> Mg2+ > K+ > Na+
In soil there are many such binary cation pairs and
binary equilibrium relations. The distribution of the various types of
cations between solution and adsorbed phase depends on many different exchange
equilibria (one for each different pair of cations) like the one above.
These include cation exchange with acidic cations
3XCa2+ + 2Al3+ <=> 3Ca2+
+ 2XAl3+
XCa2+ + 2H+ <=> Ca2+
+ 2XH+
The natural source of solution and adsorbed basic
cations is (chemical weathering of) primary minerals. The supply of these
primary minerals is limited. On the other hand, there is a continuous
supply of H+ from H2CO3 and organic acids.
Thus, if there is sufficient rainfall for leaching conditions to prevail,
basic cations tend to be depleted and replaced by acidic cations. Therefore,
the long-term tendency is toward soil acidification (loss of basic cations).
This is aggravated by the fact that exchange equilibria involving H+
and Al3+ favor replacement of basic with acidic cations. Also,
coupled this with the weathering of clay minerals to those of lower and
lower CECs, increased weathering leads to infertile, acidic soil.
Al3+, Ca2+ and H+
are the commonly adsorbed cations in humid regions. This reflects the long-term
leaching loss of basic cations and their replacement by acidic cations.
In contrast, Ca2+, Mg2+, K+ and Na+
are the commonly adsorbed cations in arid regions.
To raise or maintain fertility that otherwise is
reduced by leaching losses of basic cations and the removal of basic cations
in crop harvest, fertilizer and lime are added.
Cation Exchange Capacity
CEC is moles of positive charge adsorbed per unit
mass of soil. It is expressed
in cmolc / kg. It includes acidic and
basic cations.
CEC varies with
Types of colloids present
Amounts of these colloids
pH
Charge at pH 7
Colloid
Permanent pH-dependent
Total
cmolc kg-1
Humus
20
180
200
Vermiculite
140
10
150
Smectite
95
5
100
Illite
24
6
30
Kaolinite
0.4
7.6
8
Al(OH)3
0
4
4
Exchangeable Basic Cations
Sum of adsorbed charges due to Ca2+, Mg2+,
K+ and Na+ per kg of soil divided by the CEC (cmolc/kg)
is called the percentage base saturation (%BS). The higher
the soil pH, the higher the percentage base saturation and the lower the
pH, the lower the percentage base saturation.
Anion Exchange
Analogous to cation exchange
XSO42- + 2Cl- SO42-
+ 2XCl-
Common with 1:1 type silicate clays and Al and Fe
oxides at low pH.
The sum of exchangeable anions per unit mass of soil
is called the anion exchange capacity (AEC). It is expressed
in units of cmolc / kg.
In addition to anion adsorption at positively charged
exchange sites, certain anions may also be specifically adsorbed,
i.e., bonded to the colloid surface rather than simply attracted by electrostatic
force. This is common for phosphate, sulfate and molybdate anions.
Back to AGRO 2051
